Sanofi Pasteur announces FDA approval of Menactra® - European Pharmaceutical Review
4.6 (122) · € 23.50 · En Stock
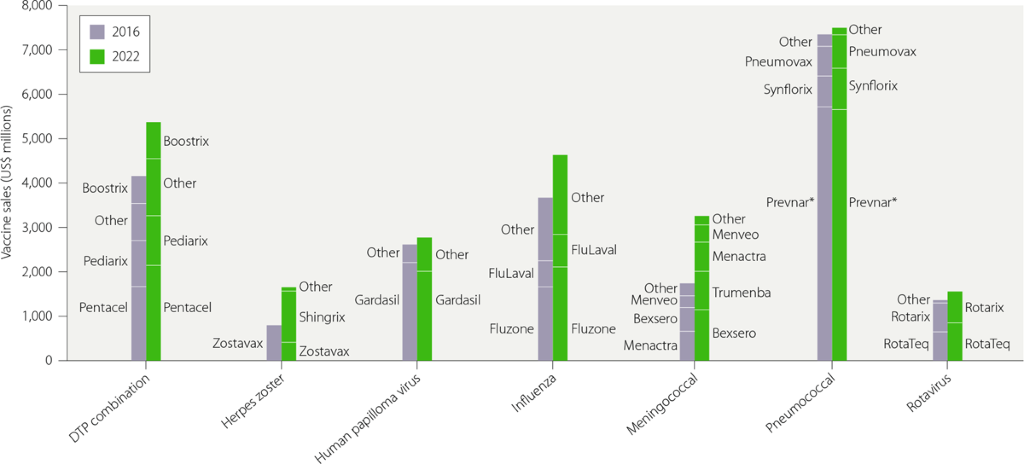
The commercial outlook for infectious disease vaccines

The status of COVID-19 vaccines in India: A review
Sanofi Access to Medicine Foundation

The status of COVID-19 vaccines in India: A review

Synthetic Glycans to Improve Current Glycoconjugate Vaccines and Fight Antimicrobial Resistance

PDF) Meningococcal Quadrivalent Tetanus Toxoid Conjugate Vaccine (MenACWY-TT, Nimenrix™): A review of its Immunogenicity, Safety, Co-Administration, and Antibody Persistence
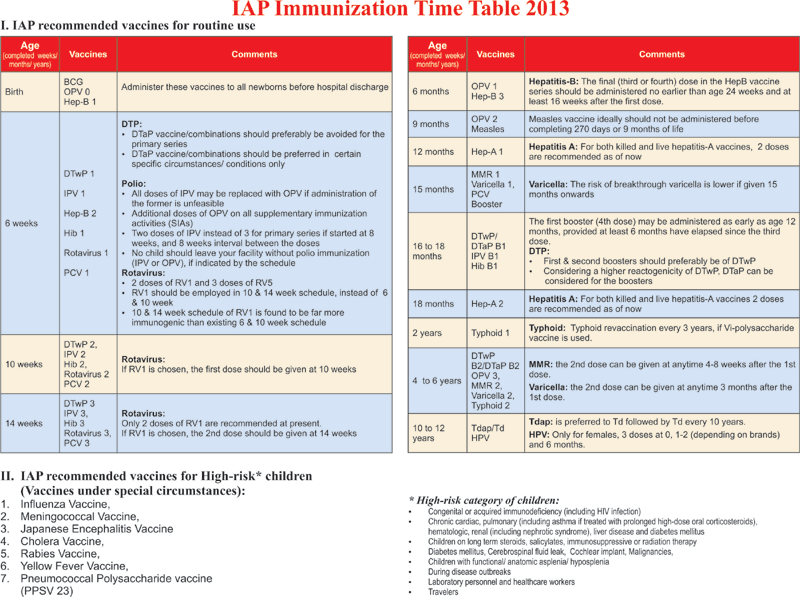
Indian Academy of Pediatrics (IAP) Recommended Immunization Schedule for Children Aged 0 through 18 years – India, 2013 and Updates on Immunization
Menactra Antitrust Direct Purchaser Class Action Settlement - Top Class Actions
Menactra®, 589-05, Vaccines & Biologics, Product Catalog

sny-20201231
Full article: Fighting Neisseria meningitidis: past and current vaccination strategies

Indian Academy of Pediatrics (IAP) Recommended Immunization Schedule for Children Aged 0 through 18 years – India, 2013 and Updates on Immunization

2019/12 – IR – Capital Markets Day

Immunization Update - Advances in Pediatrics

Menactra Meningococcal Vaccine, Sanofi Pasteur at Rs 3700/vial in Mumbai