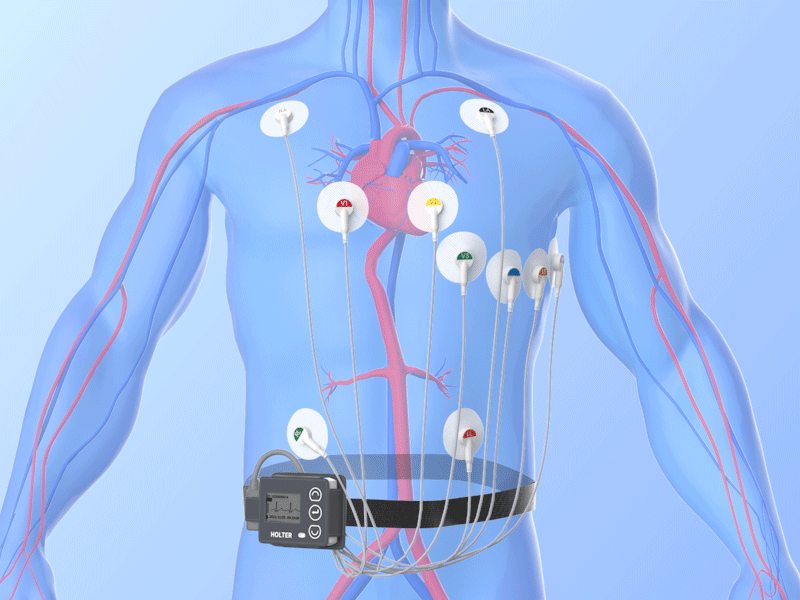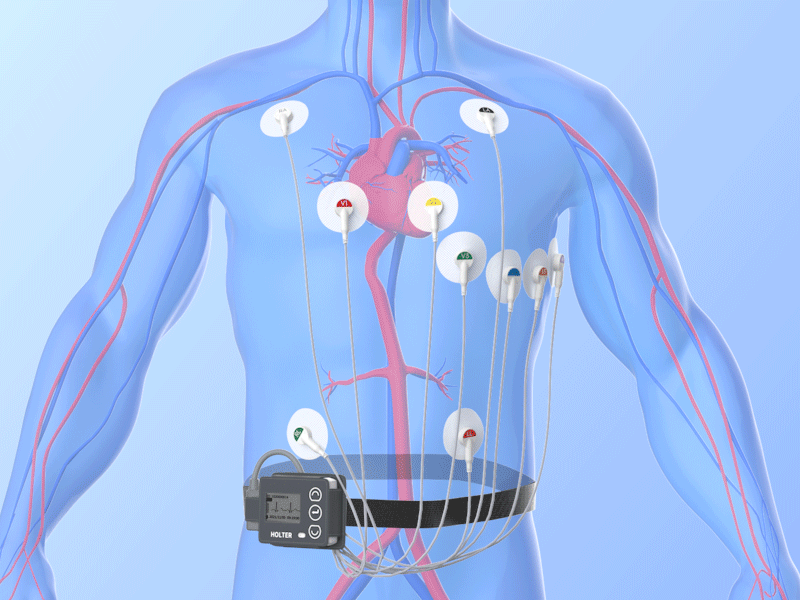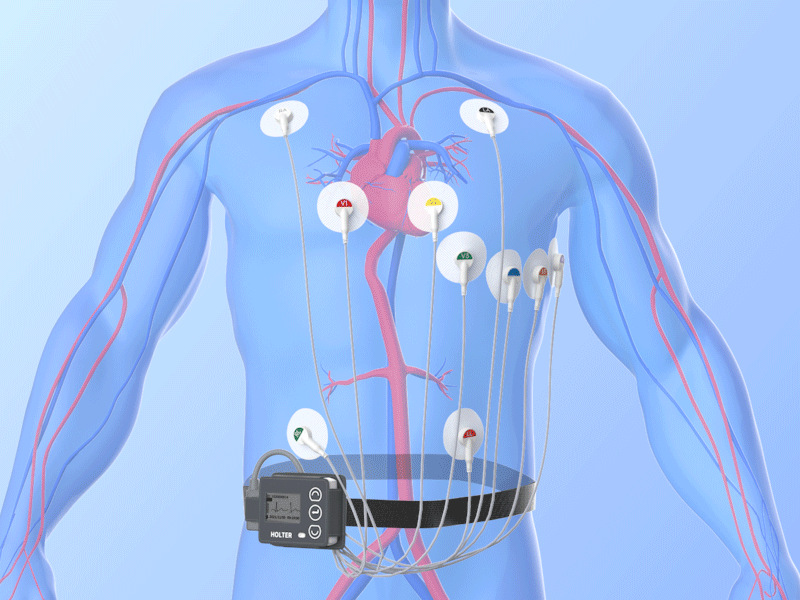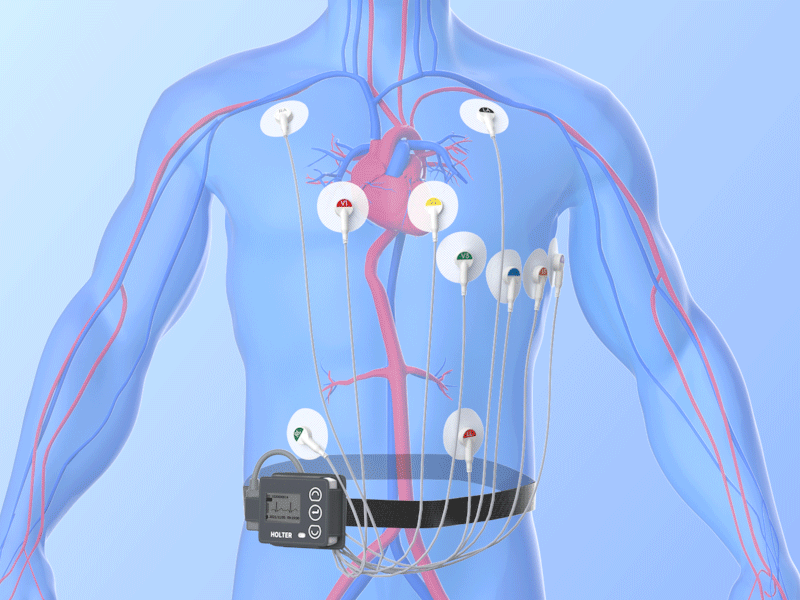
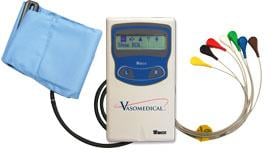
Combined Holter Records Both ECG, Blood Pressure
4.9 (129) · € 17.00 · En Stock
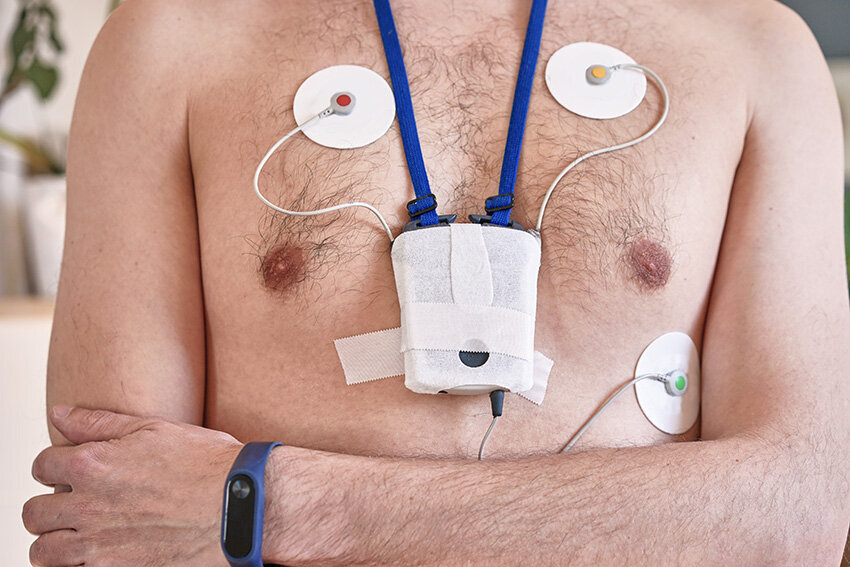
April 13, 2010 – The U.S. Food and Drug Administration (FDA) recently cleared a combined electrocardiogram (ECG) Holter and ambulatory blood pressure monitoring recorder and software analysis system. The Vasomedical-Biox Model 2301 system is among the first combined systems to simultaneously and continuously record and store ECG and blood pressure data for 24 hours. The ability to view these parameters simultaneously, side by side gives the physician more useful information to evaluate the patient's cardiovascular status. In addition to being a compact device that replaces two separate recorders, it also provides interactive blood pressure recording at times of certain cardiac abnormalities. This can enhance the diagnostic value of the recorded data, while increasing patient comfort and ease of use. For more information: www.vasomedical.com

1-channel Holter recorder - EC-3H/ABP - Labtech - 2-channel / 3-channel / Bluetooth

Ambulatory Blood Pressure Monitor NIBP Holter ABPM50 USB Software 24 H – CONTEC
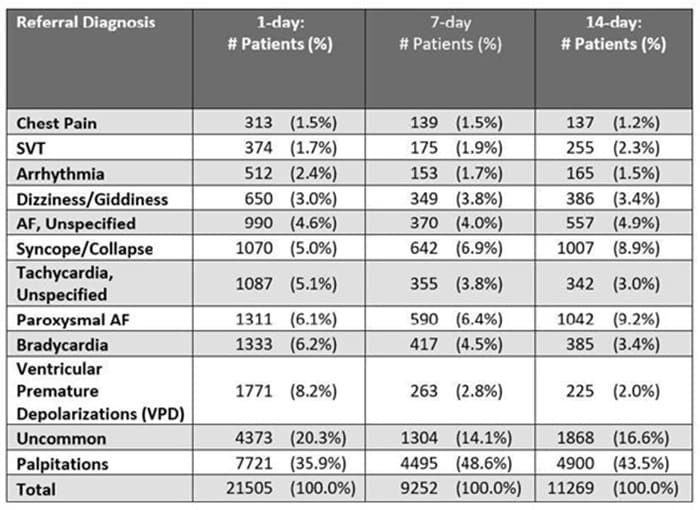
New ambulatory monitoring data at ESC - News

Electrode EKG 12 Channel Portable Recording ECG Machine Monitor 12 Lead ECG Holter - China Electrocardiograph and Portable ECG

Holter

Holter ECG - custo med GmbH - PDF Catalogs

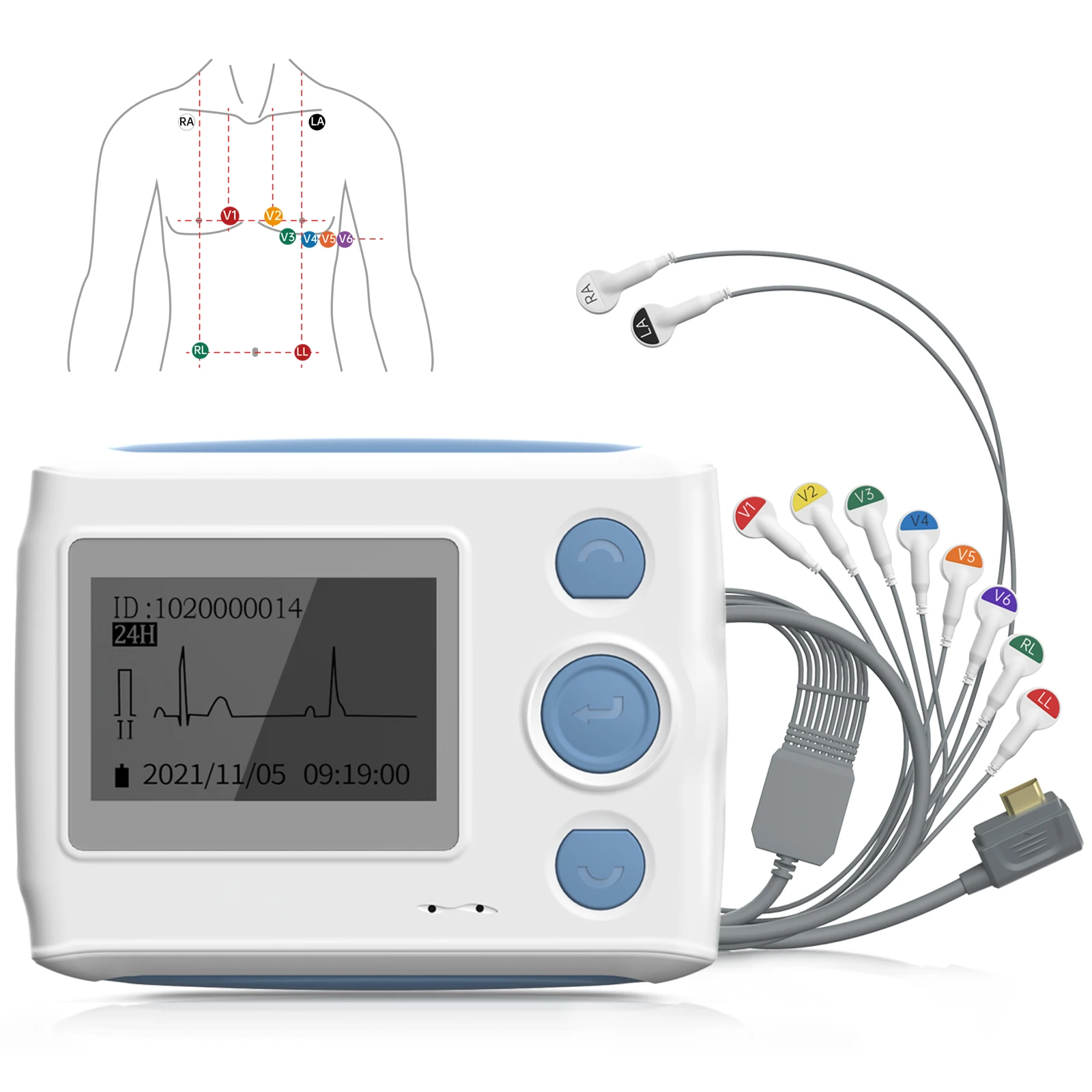
1-lead to 12-lead and exercise ECG
Combined Holter Records Both ECG, Blood Pressure
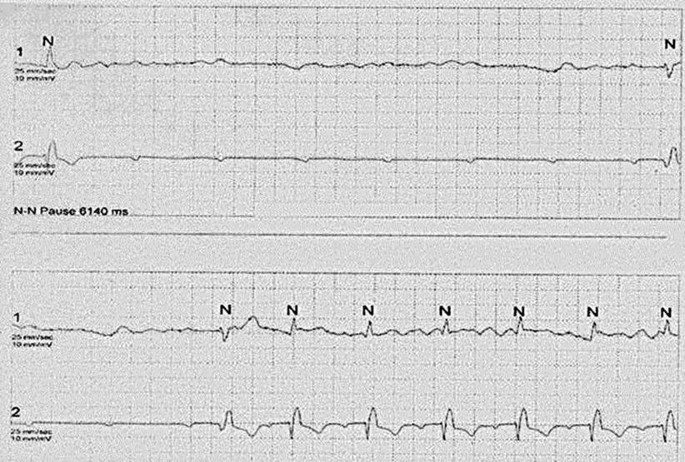
Long-Term ECG (Holter) Monitoring and Head-Up Tilt Test