- Accueil
- tpex
- PDF) A Multicenter Phase II Trial of Docetaxel, Cisplatin, and Cetuximab ( TPEx) Followed by Cetuximab and Concurrent Radiotherapy for Patients With Local Advanced Squamous Cell Carcinoma of the Head and Neck (CSPOR
PDF) A Multicenter Phase II Trial of Docetaxel, Cisplatin, and Cetuximab ( TPEx) Followed by Cetuximab and Concurrent Radiotherapy for Patients With Local Advanced Squamous Cell Carcinoma of the Head and Neck (CSPOR
4.6 (556) · € 21.00 · En Stock
A Multicenter Phase II Trial of Docetaxel, Cisplatin, and Cetuximab (TPEx) Followed by Cetuximab and Concurrent Radiotherapy for Patients With Local Advanced Squamous Cell Carcinoma of the Head and Neck (CSPOR HN01: ECRIPS Study)

PDF) A Randomized, Multicenter, Phase II Study of Cetuximab With Docetaxel and Cisplatin as Induction Chemotherapy in Unresectable, Locally Advanced Head and Neck Cancer

Targeted therapy for head and neck cancer: signaling pathways and clinical studies

PDF) A Multicenter Phase II Trial of Docetaxel, Cisplatin, and Cetuximab ( TPEx) Followed by Cetuximab and Concurrent Radiotherapy for Patients With Local Advanced Squamous Cell Carcinoma of the Head and Neck (CSPOR

PDF) A Multicenter Phase II Trial of Docetaxel, Cisplatin, and Cetuximab ( TPEx) Followed by Cetuximab and Concurrent Radiotherapy for Patients With Local Advanced Squamous Cell Carcinoma of the Head and Neck (CSPOR

Cetuximab, docetaxel, and cisplatin as first-line treatment in patients with recurrent or metastatic head and neck squamous cell carcinoma: a multicenter, phase II GORTEC study.

Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial - The Lancet

Efficacy and safety of neoadjuvant chemotherapy in resectable oral squamous cell carcinomas: A systematic review and meta-analysis - ScienceDirect
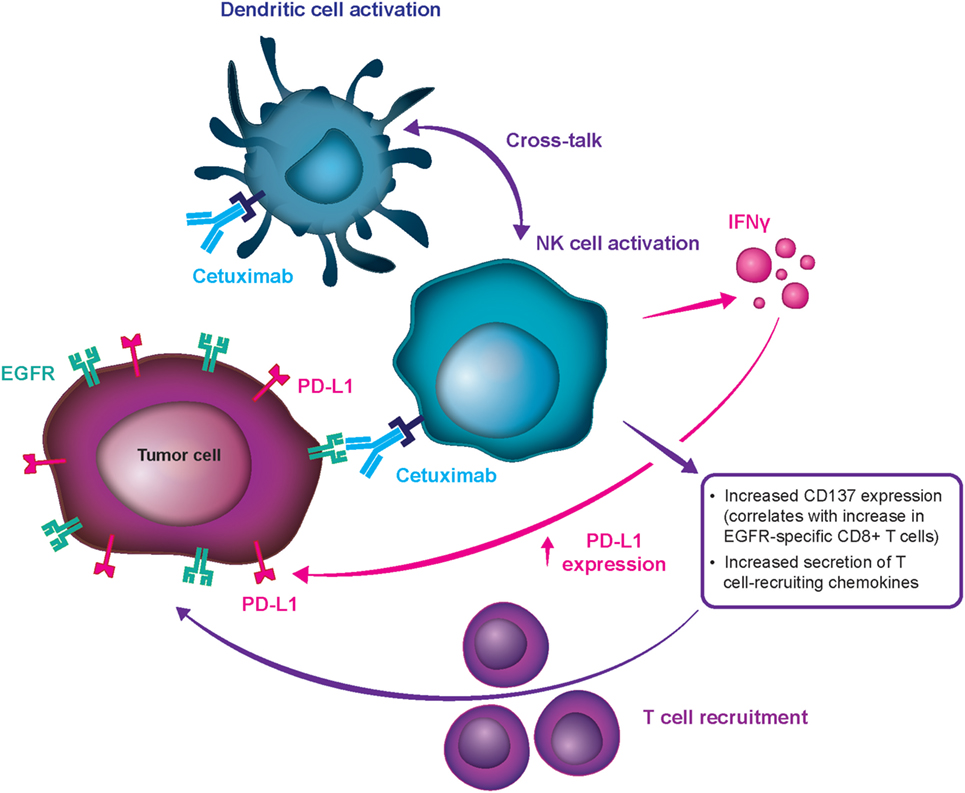
Frontiers Evidence-Based Treatment Options in Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck

Docetaxel, cisplatin and 5-FU compared with docetaxel, cisplatin and cetuximab as induction chemotherapy in advanced squamous cell carcinoma of the head and neck: Results of a randomised phase II AGMT trial











