5.85g of NaCl is dissolved in 1L of pure water. The number of ions
4.9 (246) · € 25.99 · En Stock
5.85g of NaCl is dissolved in 1L of pure water. The number of ions in 1ml of this solution is

Molarity Calculations
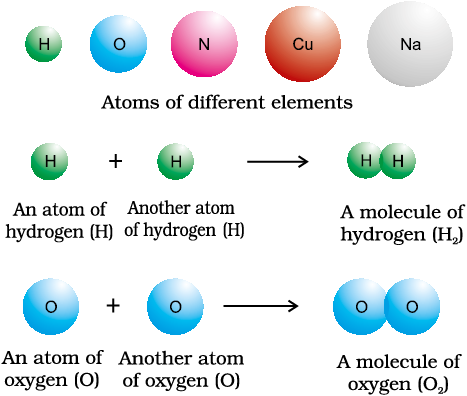
NCERT Ebook for Some Basic Concepts Of Chemistry - Some Basic Concepts Of Chemistry - Chapter 1 - NCERT Chemistry - XI

PDF) Physical Chemistry by P Bahadur abhishek kumar gautam

FINAL fiit-jee SOME BASIC CONCEPT..docx
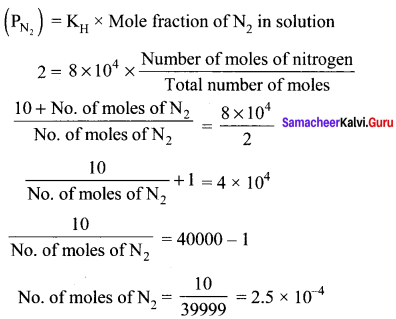
Samacheer Kalvi 11th Chemistry Solutions Chapter 9 Solutions – Samacheer Kalvi

WO2020102346A1 - Methods of treatment for cystic fibrosis - Google Patents
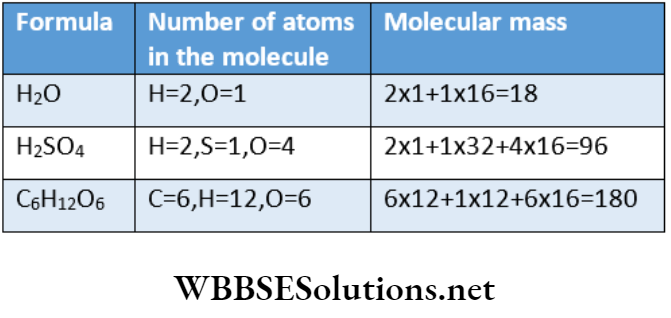
Vasantha, Author at WBBSE Solutions
5.85g of NaCl is dissolved in 200ml of water. What will be the molarity of the solution? - Quora

Bansal classes chemistry study material for iit jee by S.Dharmaraj - Issuu

2225 questions with answers in PH

Q55: In one litre of pure water, 44.4 g of calcium chloride is dissolved. The number of ions in one mL of the resultant solution is: (a) 7.23 102 (6) 7.23 1020 (C) 4.82 102 () 4.82*10** 1 Page 11 of 20
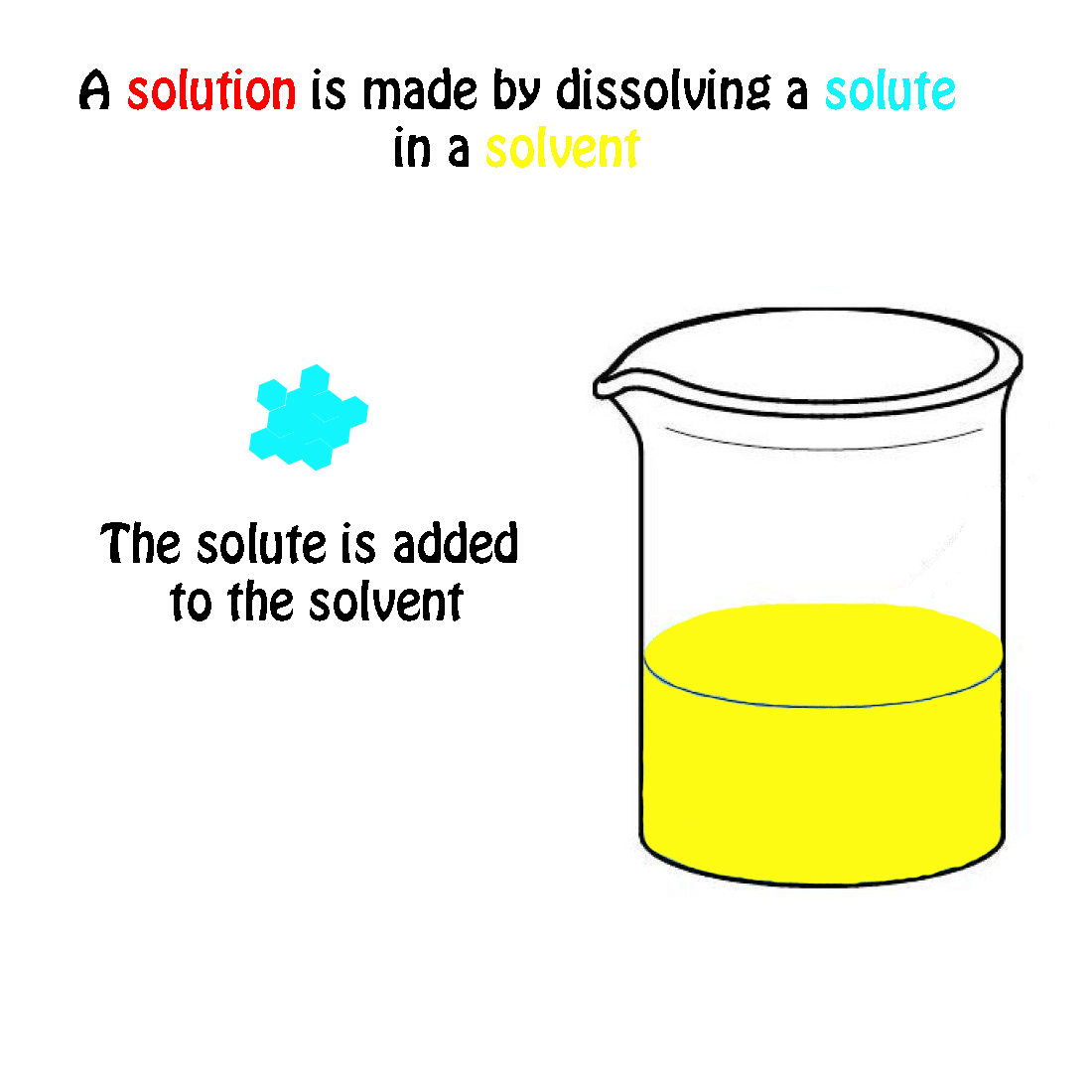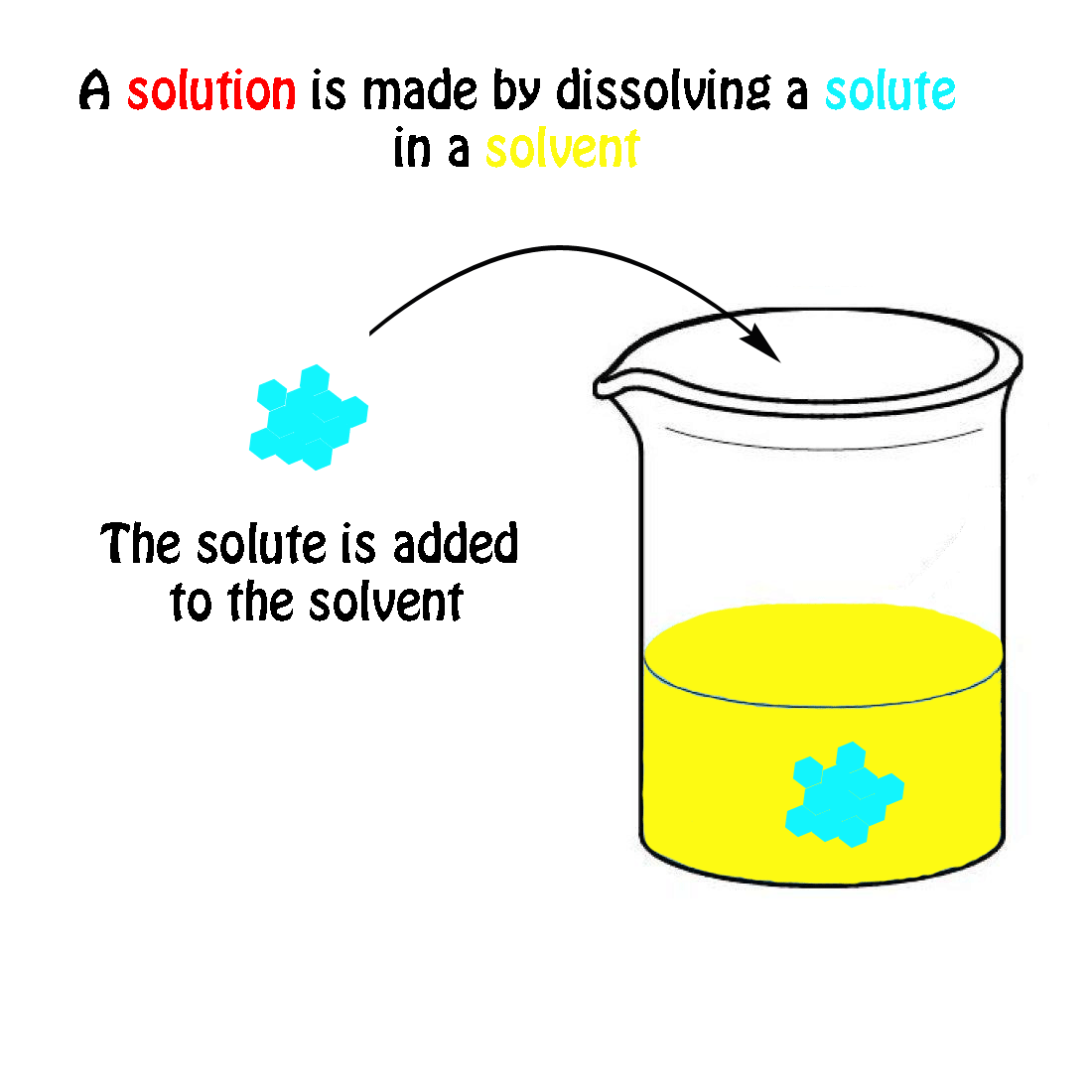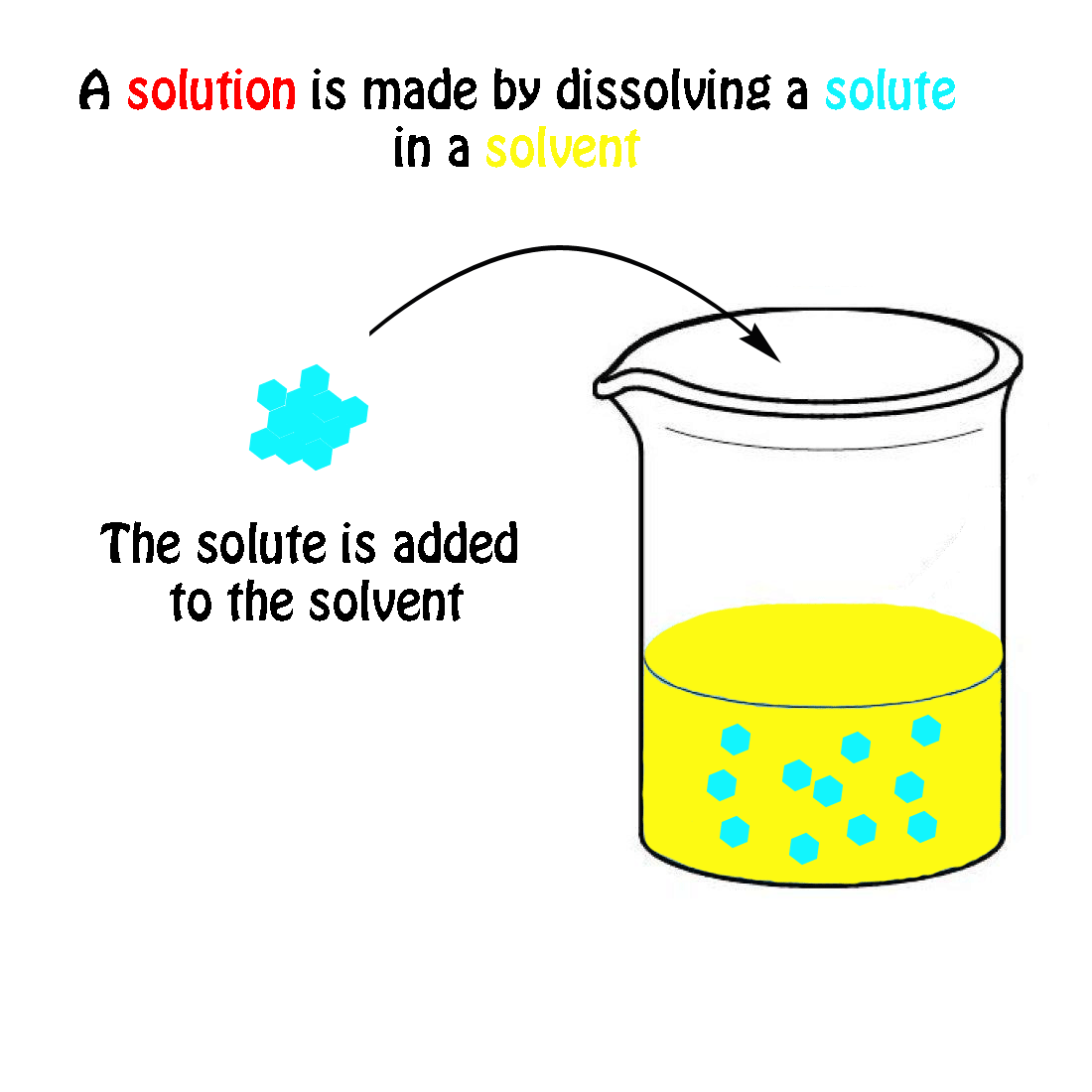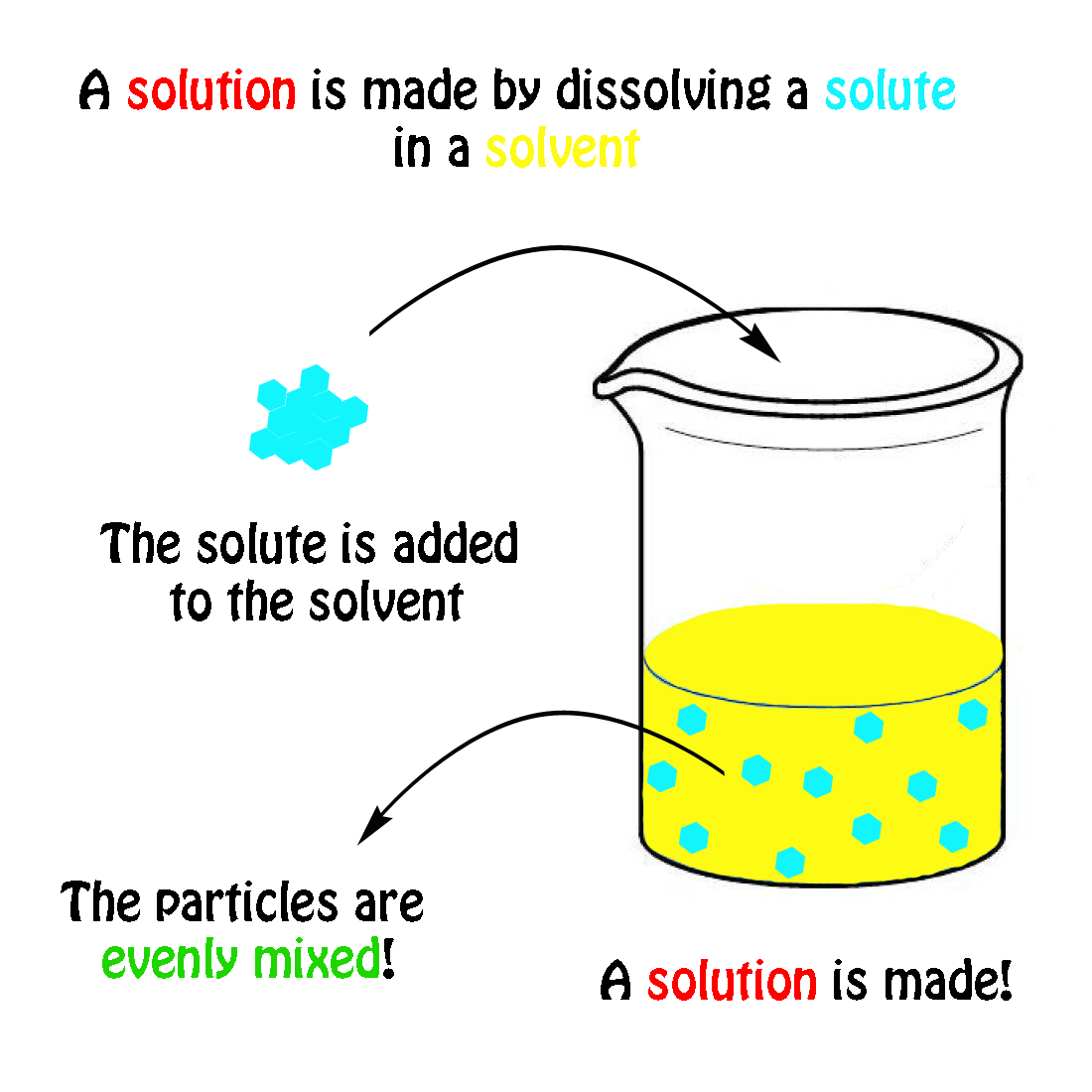
The molecular weight of NaCl is 58.44 grams/mole. If you had a 1.0 molar solution (1.0 M), you would have to put 58.44 g of salt in 1.0 liter of solution. How